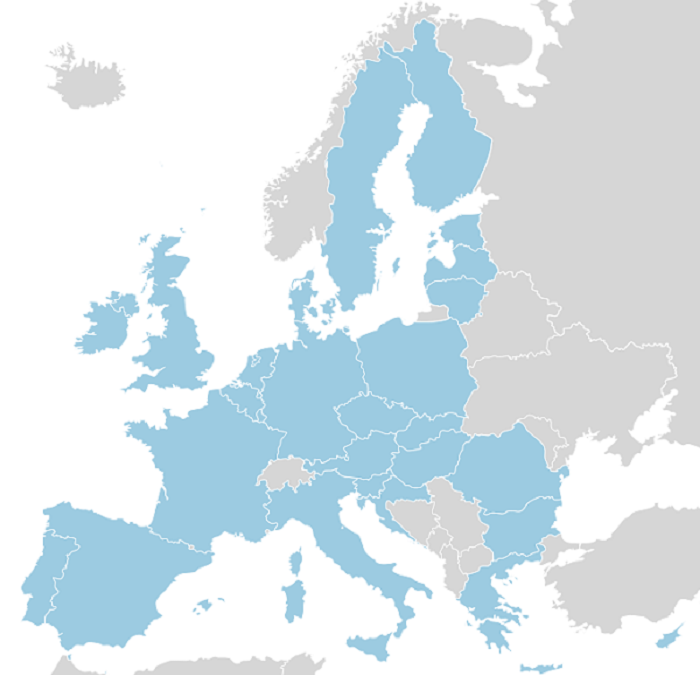
In 2019, the AKU Society’s international clinical trial, DevelopAKUre, will end. We hope that it will prove that the drug nitisinone is an effective treatment for AKU. If this happens, we will apply to a body called the European Medicines Agency (EMA) for ‘marketing authorisation’. This means that we will be legally allowed to sell nitisinone anywhere in the European Union (EU – see picture), as well as in Iceland, Liechtenstein and Norway.
In June 2016, a majority of British voters voted to leave the EU in a referendum. The British Government decided to respect this vote. It applied to leave the EU on 29th March 2017. According to EU law, the UK now has until the end of March 2019 to leave. In the meantime, the Government are trying to make a deal with the EU. Their aim is to leave the EU without disrupting jobs or security.Two weeks ago, British Prime Minister Theresa May made a
speech, in which she was clearer about the kind of deal she wanted. She said she wanted the British economy to be divided into three areas. In one area EU rules would be replaced by new British rules. In another area EU rules would also be replaced, but the UK would agree ‘common goals’ with the EU. In a third area the UK would continue to follow EU rules.She said that medicines would be part of the third area. More specifically, she wants Britain to remain a member of the EMA, despite leaving the EU. This means that the UK will vote at EMA meetings, pay into the EMA budget and – most importantly – put in place its rules. In other words, if Theresa May gets the deal she wants, we would be allowed to market nitisinone in the UK even after Brexit if the EMA lets us.The European Council, which sets strategy on the EU side, will meet next week to discuss the British plans. But many in the pharmaceutical industry are
worried by what the President of the European Council has said in the meantime. He states that the deal must not involve
‘cherry-picking’. This suggests that the UK cannot remain a member of the EMA unless it stays in the EU
‘single market’, accepting the whole system of EU law and the decisions of EU courts. This is because even if the UK puts in place EU rules on medicines, the pharmaceutical industry will be involved with other parts of the British economy which have not been regulated by the EU. He believes this will undermine the EU.Theresa May, however, says she does
not want the UK to stay in the single market. She argues that this would not respect the referendum result.As a result, we at the AKU Society are unsure about the future. The UK could choose to follow EMA decisions even if the Government are unsuccessful and the UK leaves the EMA. This would still mean that we would only have to apply to the EMA, and not to a British equivalent. However, the UK would then have no say over how the EU chooses to regulate and control the supply of medicines. They could, for example, choose to impose customs checks or taxes on medicines which flowed across the border.To make things even more complicated, Theresa May has also said that she wants the deal to include a
‘transitional period’. This would mean that the UK would stay in the single market for up to two years after leaving the EU in March 2019. The European Council seem happy with the idea, though they will decide next week. If this happens, the UK would remain a member of the EMA during this period. During this time, we would hope to get the EMA to approve nitisinone.