This November is a very important month for the AKU Society and for AKU Patients everywhere, as it marks the four year anniversary of the DevelopAKUre consortium receiving the funding needed to begin the three groundbreaking studies into a potential treatment for AKU.
Thought to be the first time a UK patient group has been the driving force behind a major international drug research trial, we are extremely proud of how far we have come and how close we are to finding an effective treatment for AKU, after striving so hard for one over the past 11 years.
This would not have been possible without our fantastic partners and those willing and pioneering patients who have travelled from all over Europe and beyond to take part in the trials.

The AKU Society’s partners form the DevelopAKUre consortium. This is made up of 12 different members that came together to share expertise and knowledge, with a shared goal of supporting successful research into AKU. It includes national AKU Societies, universities, pharmaceutical companies, hospitals and Biotech companies.
In November 2012, the DevelopAKUre consortium found out that it was successful in receiving £8 Million funding to begin the long awaited trails. This included £4.8 Million from the European Commission as part of the research funding scheme called the 7th Framework (FP7) and £3.2 million from co-financing from the other consortium members.
This allowed the consortium to proceed with the planned dosage study SONIA 1, which was designed to underline which dosage would be the most appropriate over the lifetime of a patient and to test whether nitisinone reduced the amount of Homogentisic acid (HGA) in the bodies of AKU patients.
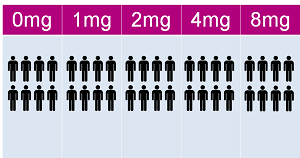
SONIA 1 lasted 4 weeks and was made up of 5 patient groups of 40 patients who took nitisinone at varying doses, starting at 0Mg to 8mg. In January 2013, recruitment opened for AKU patients to travel to one of the 2 trial sites in Piestany, Slovakia or Liverpool in the UK. The AKU Society also held a webinar to explain the trial and to encourage patients to come forward.
The first group of patients travelled to Liverpool in May 2013 and the last travelled to Slovakia in November 2015. Ultimately, SONIA 1 led to an understanding that nitisinone does indeed lower the levels of HGA in the body and that this is dependent on a higher dose of the drug. Leading us to understand that 10 Mg is the most appropriate dose, which is now being used in SONIA2.
After the SONIA 1 findings were analysed and released in February 2014, the consortium turned its attention to the ongoing SONIA 2 study.
SONIA 2 is designed to see if nitisinone slows down the damage to the body in AKU by reducing the levels of HGA, and to show that nitisinone is safe for use in AKU patients. In SONIA 2, we recruited 138 AKU Patients. These patients were then split up evenly between 69 patients who receive 10 mg of nitisinone daily, and 69 patients who do not. This ‘no treatment arm’ is crucial as it will be used to show what happens to a patient with AKU over the same time as those who are taking the drug.
SONIA 2 is split between 3 clinical sites, in Piestany, Slovakia, Liverpool in the UK and Paris, France and will involve 6 visits to one of these sites. The trial is ongoing and is currently seeing patients for their 4thvisit.
The trial is expected to finish in 2019 with the findings released soon after. If successful, the data from SONIA 2 will be presented to the European Medical Association (EMA) and will be used to license nitisinone as a treatment for AKU globally.
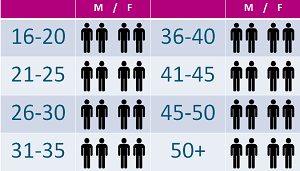
SOFIA is not testing a drug but is being used to highlight at which age the symptoms of AKU begin. This will hopefully let us know at which age it is appropriate to begin treatment for AKU. The study aims to see 32 patients at Liverpool in the UK, from ages 16 to 50 + and has been ongoing since the start of SONIA 2. We are now at the end of the study and hope to see our last patient in December this year.
The AKU Society has played a pivotal part in the groundbreaking trials above, from recruiting patients and booking flights to being tasked with dissemination, promoting the work of the consortium all over the world and running the DevlopAKUre website.
Without the funding the AKU Society helped to gain, we would be in a very different position than we are today. It’s been over 100 years since AKU was first described, and we are now closer than ever before to an effective treatment.
For more information please visit the DevelopAKUre website or contact Ciarán Scott the AKU Society’s Clinical trials officer.
