Today’s blog is written by the head of the Brazilian AKU Society, Flavia Mayrink. Originally written in Portuguese, the blog details her recent visit to the National Institutes of Health (NIH) which is based in Bethesda, Maryland, USA.In September 2018, I got in touch with Dr Wendy Introne to find out about the clinical research study at the NIH. I wanted to see if it was possible to participate as a volunteer. She sent a copy of the consent form which summarizes the studies performed at the NIH.She also informed us that the current study is a natural history study. The main purpose is to collect information from patients and perform several evaluations, with no prescribed medication.I spread the information about the study through the AKU Society Brazil and two non-identical twin brothers also volunteered to participate.

We had to pay for our tickets from Brazil to the US. When we arrived at the NIH they reimbursed our flight from our point of entry in the US to the NIH and also paid for all of the testing and booked a hotel for me and my husband. I also had a shared room at the NIH Clinical Center, where I could spend the night if I wanted.Before our visit, the NIH sent us all the information needed: a welcome letter; hotel information; a schedule of appointments; all shuttle schedules; a patient information sheet; a map of the Clinical Center; a consent for Alkaptonuria tests; and a consent for cardiac testing.
We travelled to Bethesda on Monday, May 6th. On Tuesday, we arrived at the NIH, did all the admission paperwork and were sent to our rooms on the inpatient unit on the 5th floor. Everyone was kind and everything was great. We finally met Dr Introne and she also introduced us to Dr Christina Peroutka, who was helping her with the study and made everything easy for us. They explained to us all about our appointments, how the exams worked and informed us about the objective of the study and next steps.
Each of us had a different schedule and the twins also had an interpreter available for them. The exams consisted of x-rays of the spine, shoulders, hips and knees. We also did a bone density exam. The biggest component of the study was a cardiac evaluation that included an echocardiogram (ultrasound of the heart), cardiac CT, and cardiac MRI. We also did an eye exam, an ultrasound of the kidneys looking for renal stones, and some blood and urine testing. There was also a clinical photo session.The high point of our days in the NIH was the musculoskeletal examination with Dr Monique Perry, a physiatrist specialized in musculoskeletal function. She carried out a thorough examination and explained what we should do and not do to have a better quality of life. She gave us tools to help us during this rehabilitation process (TENS unit, thera bands, hot and cold therapy gel wraps and a copy of exercises and information sheets for lower back pain and knee pain). It was the best support ever: a lot of information based on our needs and pains.Our last appointment was with Dr Introne and Dr Peroutka. They explained all the results of the exams, gave us a copy of them and told us what we could do according to our results. They also told us that they want to start a new study with medication in the future. We are looking forward to that.Everyone at the NIH was kind and supportive. The week was busy and lasted until late on Thursday. We travelled home on Friday.
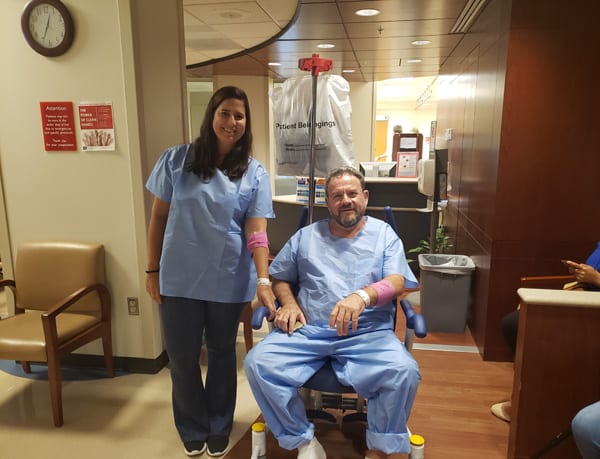

We may receive no direct benefit from taking part in this study, but to know that it may help future patients and help doctors know more about AKU is of great value to me.
We spent a lot of time during the week talking about what they know about AKU and reviewing the results of our evaluations. It was great for me and very beneficial as I learned even more about AKU and what I need to do for my health.If you would like to get involved in the NIH study and have a diagnosis of AKU, you can read
more information via clinicaltrials.gov. The study identifier is NCT00005909. Alternatively, you can email
