On the 18th January, the last SONIA 2 (Suitability of Nitisinone In Alkaptonuria 2) patients were seen at the Royal Liverpool and Broadgreen University Hospital. This means that the patient element of the SONIA 2 clinical trial is now officially over.Patients from all over Europe came to Liverpool, as English and Scottish patients were excluded from the study because they already attended the National Alkaptonuria Centre (NAC) also based in Liverpool.All three clinical trial sites (Liverpool, Paris and Slovakia) are now finished and the long task of analysing the data has started in order to see if nitisinone does reduce the amount of HGA, the acid that cause the damage in AKU patients’ bodies. Once finished and if positive, we will present the evidence to the European Medicines Agency (EMA) for a license for nitisinone to treat AKU. We hope that this will mean the drug will then be available to everyone across Europe and beyond.

Prof Ranganath, SONIA 2 chief investigator, said:“Being part of the SONIA 2 clinical trail was a real privilege. I was delighted to be part of the study and meet such a great group of patients here in Liverpool. People from all over Europe gave up their time to travel many miles to Liverpool to help future AKU patients and expand our knowledge of the disease.“I really valued working with people where my contribution will hopefully make a difference to their lives. The patients are such a mixed and interesting bunch of people who value what we are doing for them. It makes it all worthwhile. When we are successful in getting the European Medicines Agency to license nitisinone for people with AKU, it will bring hope to thousands of people that there is a disease modifying treatment for this condition for the very first time. I absolutely love working in the DevelopAKUre consortium as this is a fantastic and enthusiastic group of people who have come together and dedicated themselves to ensure that nitisinone treatment becomes available to all.”
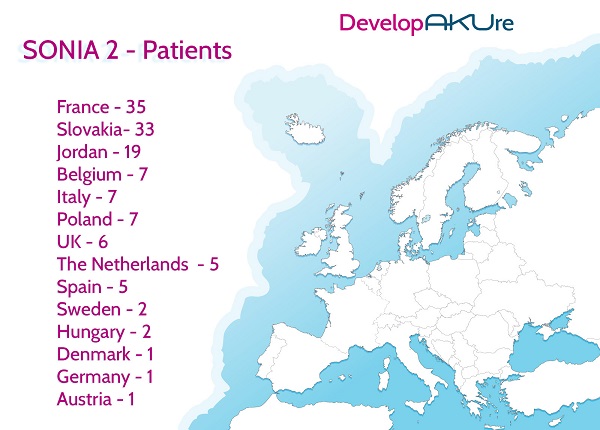
The DevelopAKUre consortium would like to thank every patient who was part of SONIA 2, SOFIA and SONIA 1. Please continue to check out the blogs for the latest updates on the progress of the DevelopAKUre consortium and nitisinone.
